Alta Metabolome Testing Inc
Alta Metabolome Testing Inc
Beverage testing is essential to understand the parameters that affect or influence product qualities for e.g. fermentation methods, terroir, grape variety, sensory profile, anti-oxidant, nutrient and contaminant profiles, aging, storage etc. Consistent quantitative measurements of contaminants and other chemicals responsible for the flavors and faults can minimize batch-to-batch variations in test, aroma and quality of the beverages. This can have a significant impact on both the human health and the overall sales.
At AMTI we are committed to deliver comprehensive analytical results on alcoholic beverages (wine and beer) and envision to expand our services to the analyses of juice, milk and other beverages in the near future.
View our ServicesServices
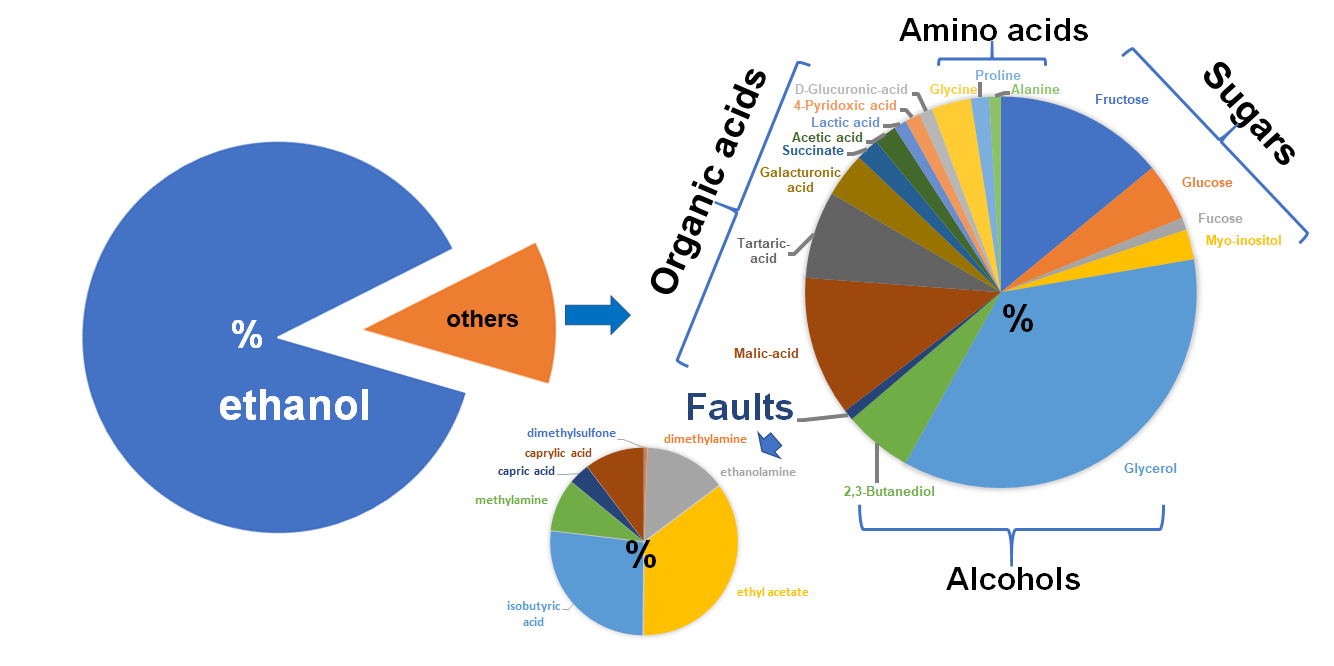
Our comprehensive beverage testing technology covers a wide range of compounds responsible for flavor, taste, odor and faults in the samples. Our state-of-the-art method includes NMR, LC-MS, and ICP-MS based extensive analyses in combination with completely automated data processing platforms and all required metabolite databases. We generate accurate and consistent results in a very time-efficient manner and therefore our services are very cost-effective as well. Our analytical results will give insights to the following areas:
Various Classes of Compounds
The analytical results, generated in an ISO 17025 certified lab, will cover the following classes of compounds:
- Amino acids (glycine, proline, glutamic acid etc.)
- Sugars (glucose, fructose, total sugar content etc.)
- Smoke taint (guaiacol, guaiacol glucoside etc.)
- Organic acids (malic acid, tartaric acid etc.)
- Other alcohols (glycerol etc.)
- Faults (methanol, ethyl acetate, isobutyric acid etc.)
- Herbicides and pesticides
- Heavy and toxic metals (Arsenic)
Adulteration of Beverages and Toxic Compounds
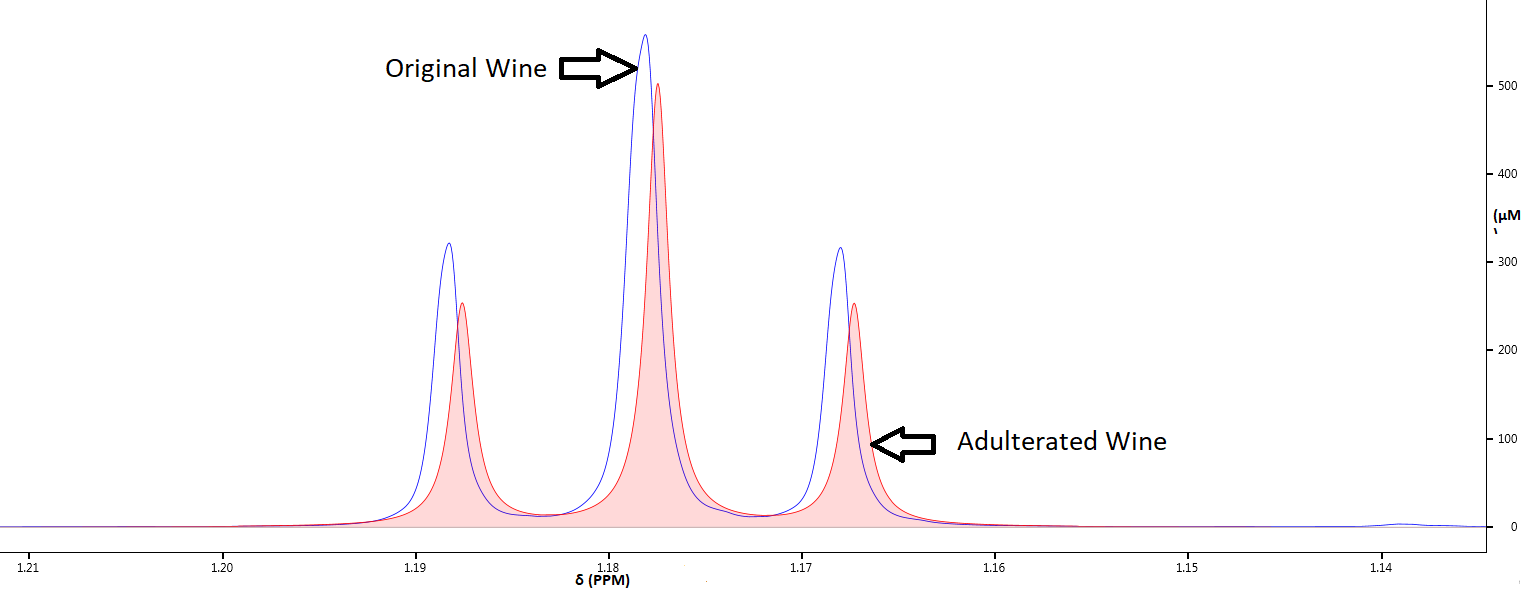
Given the very high market value of the beverage industry, it is extremely important to prevent fraud. Our quantitative NMR platform helps authenticate samples by generating fault profiles.
Methanol is a toxic substance that must be strictly controlled. The levels of methanol found in grape wines are similar to those that may be found in many freshly squeezed and unpasteurized fruit juices if they are stored for a period of time after squeezing. For instance, typical ranges for methanol found in Australian wines were 60–280 mg/L in reds (mean 170 mg/L) and 40–120 mg/L in whites (mean 58 mg/L). Our analysis of NMR spectra below provides evidence of adulteration in a red wine by comparing two different samples of the same type.
Analyses and Quantification of Sugars
Sugars are more common in wine than in beer. Glucose and Fructose are the most abundant ones. Sugar content in wine can vary from 1 g/L (very dry) to 90 g/L (dessert wine). We can quantify individual sugars or can measure total sugar content using NMR.
Analyses and Quantification of Amino Acids and Organic Acids
Amino acids add flavor and bitterness to wines. Organic acids add tartness and acidity which must be balanced with sweetness for the optimal taste. Too little amount of acids will lead to flat taste. Our MagMet NMR library will quantify all the abundant and relevant organic acids.
Alcohol Content
Alcohol content is an important parameter to measure to track batch-to-batch variations and to minimize the chances of adulterations. Our quantitative NMR measurements can quickly generate the ethanol content of the sample which can be compared with the expected value to check if a diluent is added. Our spectra demonstrate how the ethanol level is reduced in a wine sample that has been diluted.
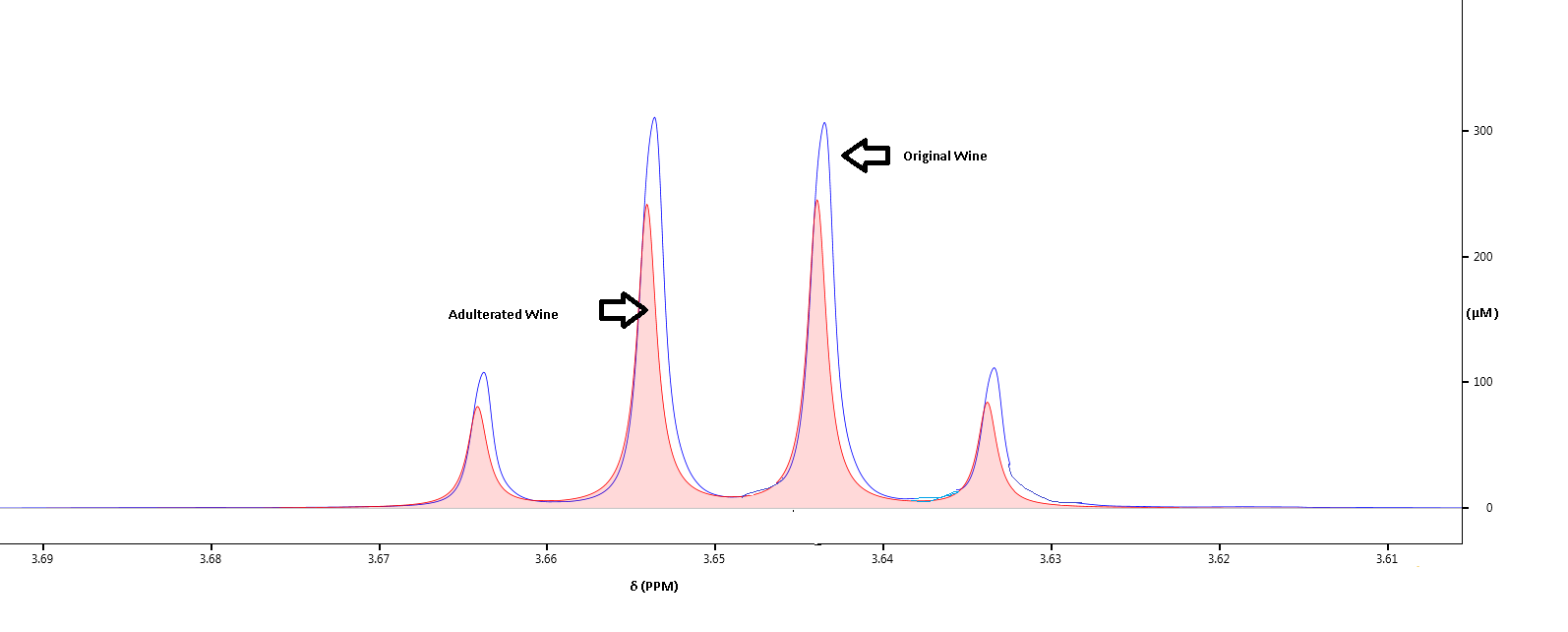
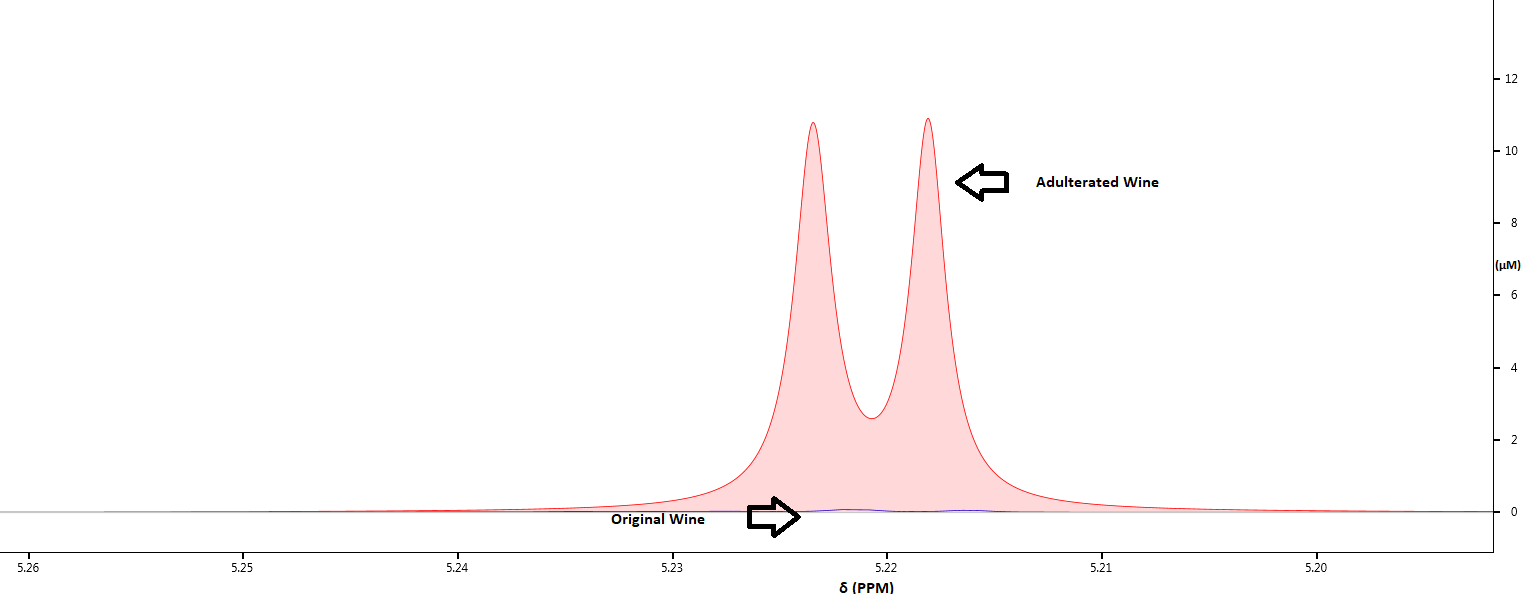
Batch to Batch Variation
We generate the “NMR fingerprint” of the samples to ensure consistency between various batches of the samples in terms of quality, taste and flavor. The complete spectral profile will indicate even minute changes in alcohol, acid and sugar contents, so that preventative measures can be taken to minimize variations. The spectra below reveal how the glucose levels differ in two samples of wine that belong to the same brand.
Bacterial Contamination
Although less common, bacterial contamination during the fermentation process can be a potential problem in the beer industry. Only a few bacteria can grow in beer and can impact flavor or result in sediment and acidity. Lactobacillus, a Gram-positive bacteria for e.g. produces excess lactic acid in beer leading to a sour taste and can be easily quantified by NMR.
NMR Analyses
We can automatically identify and quantify 61 different compounds in wine samples using our automated platform MagMet and 146 using Chenomx.
MS-based Analyses
Our LC-MS assays will quantify various classes of compounds that are below the detection limit of NMR or are structurally too complicated to be detected and quantified by NMR. These will include some sugars, amino acids, organic acids, polyphenols, biogenic amines, vitamins, lipids etc.
Lipids
Lipids comprise 2.2%–2.5% of the dry substance of barley and 2-12% of dry matter of yeast cells. Although lipids are not expected in beer or wine, they have negative effects on aging stability and foam stability of the beverages. Various fatty acids, lipids, phospholipids, sphingomyelins, and terpenes will be quantified by LC-MS.
Biogenic amines, vitamins and polyphenols
Thiamine-enriched (Vitamin B1) alcoholic beverages can be particularly appealing to the people suffering from Wernicke-Korsakoff Syndrome (WKS). Histamine and tyramine (biogenic amines), present in red wines, cause migraine to many consumers. Our comprehensive LC-MS analysis platform will cover both of these classes of compounds. Both flavonoid and non flavonoid classes of polyphenols are quite abundant in red wines and they have antioxidant properties. Resveratrol, a polyphenol, shows cardioprotective and chemopreventive effects. It also exhibits anti-inflammatory, antibacterial, antifungal, antiviral, neuroprotective, antiproliferative and anti-angiogenic activities. Therefore, analysis and quantification of polyphenols is very essential in wines and achieved by our LC-MS method.
Contaminants and faults
The contaminants and faults have catastrophic effects on quality, flavor, aroma and taste and could lead to potential health risks. The LC-MS assays will also quantify various beverage contaminants and faults for e.g., herbicides, pesticides, antibiotics, toxins and volatiles (smoke taint), cork taint, phenolic taint. We aim to analyze >500 compounds overall in beverages using LC-MS.
Compounds causing unpleasant odor
Some sulfur containing compounds or amines can lead to burnt and fishy odor to wine samples. We can detect very low concentrations of these small molecules for e.g., dimethylsulfide, dimethylsulfone, dimethylamine, ethanolamine etc.
Degradation parameters
We detect various compounds that indicate degradation of wine samples for e.g., acetic acid, acetone, ethyl acetate, furfural etc.
Heavy and toxic metals
Our ICP-MS assays will quantify several metal contaminants, for e.g. Arsenic (dimethylarsinate, monomethylarsonate, arsenite, arsenate). Metals in wine are required for healthy growth of grapes and can also affect the look, taste and aroma of wine. We aim to analyze 40 metals in beverages using ICP-MS.
We use LC-Autofit (a completely automated LC-MS data analysis platform developed in Dr. Wishart’s group), which significantly reduces the time required and therefore cost of the analyses.
Knowledge & Technology
Metabolomics
Metabolomics is the study of metabolites (small organic molecules generated via metabolism) and the field has become increasingly popular over the last two decades because of its widespread application in clinical and biological research. The study involves quantitative measurements of thousands of metabolites using various analytical techniques.
Dr. David Wishart, founder of The Metabolomics Innovation Centre (TMIC, https://metabolomicscentre.ca ) is one of the pioneers in the field of metabolomics. He has led the Human Metabolome project which resulted in the development of Human Metabolome Database (HMDB, https://hmdb.ca ), a thorough and comprehensive database for human metabolites. The database contains clinical data obtained by extensive spectroscopic studies on biological samples. The HMDB is also associated with a few other databases, for e.g., Drugbank (drug metabolites, https://go.drugbank.com ), T3DB (toxins and pollutants, http://t3db.ca ), FooDB (food components and additives, https://foodb.ca ), etc.
Historically, GC-MS was the first technique used in the field of metabolomics. Amongst other techniques, LC-MS is the most commonly used method because of its high accuracy and sensitivity. NMR was first used back in 1974 for the detection of metabolites in biological samples. With the improvement in the NMR spectroscopic methods and the use of higher magnetic field instruments, the NMR method has now become quite popular in the field of metabolomics as a cost-effective and time-efficient technique.
Wine & Beer
Wine is one of the most consumed alcoholic beverages worldwide and an important part of the Mediterranean diet. Preliminary studies found that drinking small quantities of wine (up to one standard drink per day for women and one to two drinks per day for men), particularly of red wine, may be associated with a decreased risk of cardiovascular diseases, cognitive decline, stroke, diabetes mellitus, metabolic syndrome, and early death. Other studies found no such effects.
Early food and beverage testing focused on measuring pH, fat, sugar, protein and/or alcohol content using standard wet chemistry or manually intensive approaches. Even today most beverage testing uses hydrometers (density testing), pH paper and simple “wet” tests for certain acids or fats. More extensive testing for vitamins, contaminants and polyphenols content started emerging in the 1980s with the appearance of spectrophotometry, chromatography/separations, and liquid chromatography mass spectrometry (LC-MS) as well as nuclear magnetic resonance (NMR) spectrometry. However, these “high-end” tests were typically limited to specialized labs measuring a small number of compounds (10-15) and would often cost hundreds of dollars. Immunosorbent tests such as ELISAs for testing for drugs, antibiotics and toxins started to emerge in the late 1990s and early 2000s. These tests also cost hundreds of dollars and can take hours for the detection of just one or two compounds. The development of metabolomics in the early 2000s led to a significant improvement in the ability to detect and measure hundreds of compounds in foods and beverages.
Only a few bacteria can grow in beer. Higher the hop rate, lower the bacterial growth; hop resins from hop flowers are added to control bitterness of beer. Other reasons for low bacterial growth in beer are high ethanol concentration, low pH, lack of oxygen during the brewing process, limited sugar and temperature. α-acids and β–acids are the main components of hops with the former being mainly responsible for the bitterness; humulone, cohumulone, adhumulone, posthumulone and prehumulone are the main α-acids. Hops are added to control the bitteness and add fruity aroma to beer. Some Gram-positive bacteria present in beer are: Lactobacillus (produces lactic acid, acetoin and diacetyl, the last one being responsible for the buttery flavour), and Pediococcus (diacetyl production). Some Gram-negative bacteria are Acetic Acid Bacteria (Acetobacter and Gluconobacter, the former causes oxidation of ethanol to acetic acid to create vinegar flavor and the later causes pellicle on the beer surface, oxidizes ethanol to acetic acid and oxidizes sugars to gluconic acid), Obesumbacterium proteus (increases the level of dimethyl sulfide, dimethyl disulfide and diacetyl in beer), and Zymomonas (increases the level of acetaldehyde and hydrogen sulfide). Oxygen-free brewing condition will minimize the bacterial contamination by acetic acid bacteria.
Beer is one of the oldest and most widely consumed alcoholic beverages in the world, and its production involves a complex biochemical process of fermentation by yeast. Although the basic principles of fermentation are well established, the chemical composition of beer is still not fully understood. Recent advances in analytical techniques have enabled scientists to identify and quantify many different chemical compounds that affect the flavor and aroma of beer. Their study reveals the chemical diversity and complexity of beer and its relationship with the brewing process, the geographical origin, and the style of the beer. Beer is a complex mixture of water, carbohydrates, hops, yeast, and various chemical compounds that determine its sensory properties. The carbohydrates are derived from malted barley and are hydrolyzed into simple sugars during the mashing process. The sugars are then fermented by yeast, which produce ethanol, carbon dioxide, and other metabolites. The hops are the flowers of Humulus lupulus, which contain over 440 essential oils and bitter acids that contribute to the aroma and flavor of beer. The water is the main ingredient of beer and its mineral composition can affect the yeast metabolism and the beer quality. Recently, scientists performed a comprehensive chemical characterization of 467 beers from 40 countries. They identified at least 7,700 different chemical formulas and tens of thousands of unique molecules in beer, many of which have not been reported before.
NMR Spectroscopy
Early food and beverage testing focused on measuring pH, fat, sugar, protein and/or alcohol content using standard wet chemistry. In modern day analytical chemistry, several techniques are available to analyze small molecule components present in beverages. These include AAS, ICP, IR, UV, MS and NMR. NMR spectroscopy is an extremely powerful, accurate and reliable quantification method and requires very simple sample preparation steps. Although less sensitive as compared to mass spectrometry, NMR is non-destructive and does not require time consuming chromatographic steps. The 1D and 2D 1H and 13C-NMR spectroscopic methods for e.g., COSY, HMBC, HSQC, and TOCSY are quite popular for targeted and untargeted beverage profiling. Targeted quantitative metabolomics studies based on 1D 1H-NMR spectral deconvolution using spectra of reference compounds of known concentrations were reported first in early 1990s. NMR analyses have particularly become extremely time efficient after the introduction of automated spectra processing and profiling platforms for e.g., Bayesil, MagMet (both developed by Dr. Wishart’s group) etc.
 MagMeta completely automated platform for
MagMeta completely automated platform for
the 1H NMR spectra processing and profiling
the 1H NMR spectra processing and profiling
Manual analyses of complex NMR spectra (for e.g. spectra of wines) can be extremely time consuming (30-60 minutes per spectrum) and can introduce inconsistency due to operator’s bias. To help overcome this problem, Dr. Wishart and his team have designed MagMet (https://magmet.ca ), a web-based server which is capable of performing fully automated 1H NMR spectra processing and profiling (acquired from Varian and Bruker instruments). MagMet performs automatic Fourier Transformation, phase correction, baseline optimization, chemical shift referencing, water signal removal, and peak picking/peak alignment. MagMet uses the peak positions, linewidth information, and J-couplings from its own standard metabolite 1D 1H NMR reference spectral library to identify and quantify compounds from experimentally acquired 1D 1H NMR spectra of samples. The application of MagMet in metabolomics for the human serum, plasma, fecal samples and alcoholic beverages are reported by Dr. Wishart’s group. The optimized algorithm allows MagMet to detect and quantify 58 serum/plasma metabolites with the effective speed of 2.6 minutes per spectrum and up to 61 wine/beer metabolites with the speed of 15 minutes per spectrum.
Smoke Taint
Smoke taint refers to a specific type of wine fault. When wine grapes are exposed to heavy smoke resulting from wildfires, the produced wine may contain unpleasant and undesirable smoky flavor. Smoke tainted wines can have a negative impact in the wine industry, the effect being more adverse on red wines. The wildfires in the US, Canada and Australia in the last decade pose a big threat to the wine industry. Therefore testing of wine for the smoke influence is necessary. The markers for smoke taint are volatile small phenolic compounds that are produced via thermal degradation of lignin when wood is burnt, for e.g. guaiacol, 4-methylguaiacol, 4-vinylguaiacol, syringol, methylsyringol and cresols etc. These can either be absorbed directly by grapes or can be masked after binding to grape sugars producing glycosides for e.g., guaiacol glucoside. These glycosides, although have no smoky aroma, can be hydrolyzed at wine acidic pH and release the smoke taint markers. These markers can assess whether grapes have been exposed to smoke by comparing their concentrations to natural concentrations found in non-smoke-exposed grapes. Guaiacol or other smoke related compounds can be found in wines even without any smoke exposure. They can be formed via degradation of phenylpropanoids (a class of compounds synthesized by plants from Phe and Tyr) under acidic conditions. Quantitative analyses of these volatile phenols are performed by GC-MS.
Compound Classes Present in Alcoholic Beverages
- Organic acids and amino acids (provides flavor, bitterness, acidity)
- Sugars (provide sweetness or fruitiness)
- Phenolic compounds (control organoleptic attributes such as color, mouthfeel, astringency)
- Alcohols (ethanol adds sweetness, bitterness)
- Faults and contaminants (adulterants, smoke-bourne compounds, herbicides, pesticides etc.)
- Heavy metals (arsenite, arsenate)
Mass SpectroscopyLC-MS, GC-MS and ICP-MS
LC-MS and GC-MS are the most commonly used techniques in the field of metabolomics because of their high sensitivity and widespread compound coverages chemical derivatization and isotopically labeled derivatizing agents or internal standards.
About Us
AMTI (Alta Metabolome Testing Inc.) is a spin-off company from TMIC (The Metabolomics Innovation Center (www.metabolomicscentre.ca ), Canada’s national metabolomics core facility and led by Dr. David Wishart) and specializes in beverage testing. We are headquartered in Edmonton, Alberta, Canada. Our technology is based on NMR, MS & machine learning and provides a comprehensive metabolomic profile of beverages.
Mission
The global beverage industry is worth >US$2 trillion/year, with the non-alcoholic beverage industry contributing ~$1.1 trillion/yr and the alcoholic beverage industry contributing ~$900 billion/yr. Given their enormous market impact, the production of safe, high-quality, high-value beverages has long been a central pillar of the food industry. Our mission is to bring beverage testing to the 21st century using our extensive knowledge and several years of expertise in analytical chemistry, metabolomics and artificial intelligence.
Our Values
Customer
At AMTI, customers are our highest priority. Consumers want high-quality, good tasting drinks, regulators want safe, unadulterated, contaminant-free beverages, and producers want consistent, high-quality, high-value products. Inadequate knowledge of beverage constituents can impact human health. Foodborne illnesses (about 30% arise from contaminated beverages) affect 4 million Canadians/yr. Various contaminants such as arsenic, lead, cadmium, bisphenol A, antibiotics etc. (which tend to be more concentrated in beverages than food) contribute to many chronic health conditions such as immune disorders, infertility, dementia, Parkinson’s disease etc. On the other hand, improved knowledge of key micronutrients (vitamins, anti-oxidants) that can be found or elevated (through selective breeding or processing) in certain beverages, could enhance their health claims. Our NMR and MS-based technology will ensure authenticity and quality of the products.
Knowledge and Technology
Chemical and nutritional beverage testing, particularly for alcoholic beverages, is often minimal and uses slow and expensive technologies. Of the 10,000+ compounds in beverages, <0.1% are routinely identified and measured. Therefore, producers, regulators and consumers must often rely on gross measures (color, taste, odor) to make important production, value, health and safety related decisions. Lack of knowledge of key compounds responsible for taste features and faults, bacterial contamination, aroma or mouth feel contributes to ongoing problems with beverage production and beverage taste consistency. Therefore, this can have catastrophic consequences on sales, product valuation or consumer satisfaction. These quality control issues cost Canadian beverage producers billions in lost sales or product recalls.
We propose to significantly advance the field of beverage testing using our metabolomics-based technology. Our metabolomics databases give us the required knowledge of the compounds that are essential to be measured and why. Our high end NMR and MS instruments acquire data with very high accuracy and precision and are extremely sensitive (we can achieve a very low limit of detection or LOD). Our AI technologies also allow these data to be acquired faster, cheaper and presented in a format that is far more understandable and actionable.
The Team


Honeya Shahin
Honeya Shahin’s background includes synthetic, computational, physical, analytical, and material chemistry, obtained from course and practical work. She has a great deal of laboratory research experience working at the University of Alberta, involving synthesis, isolation, characterization and structure determination of a wide variety of metabolites and metabolite derivatives. During her research she applied nuclear magnetic resonance (NMR) spectroscopy, a high-resolution analytical technique, to characterize the metabolites in serum, fecal, wine, and beer samples. She followed a standardized protocol for experimental design, sample collection and preparation, spectra acquisition and processing, metabolite identification and quantification, statistical data analysis, and data interpretation. Honeya was part of the group that created python packages for automated NMR data processing (MagMet). These packages had several functions, such as importing time-dependent data, performing Fourier Transform, correcting phase and baseline, calibrating reference, and exporting JSON. They developed new algorithms to find and measure clusters (Features) in NMR datasets and used powerful optimization and regression methods to quantify metabolites accurately. They successfully built a complete workflow for NMR data processing and profiling that minimized human error and ensured consistent results.


Dr. Dipanjan Bhattacharyya
Dr. Dipanjan Bhattacharyya earned his PhD from the Department of Chemistry, University of Alberta in 2012 in the field of Synthetic Organic Chemistry. He worked in the field of total synthesis of natural products, synthetic methodology and drug designing. He has worked for TMIC (The Metabolomics Innovation Centre) as a Research Associate for more than five years. His main research is focused on assay developments for the detection of metabolites in bio-fluids. He has developed quite a few novel analytical techniques using ion-exchange chromatography for the detection of biomarkers (CRC, MSUD, PKU etc.) in human urine and serum samples and designed metabolomic kits to perform those assays. He has more than 17 years of experience in 1D and 2D NMR spectroscopy. Dr. Bhattacharyya’s expertise in NMR includes characterization of small molecules, identification of reaction intermediates, determination of the stereochemistry of diastereomers, characterization of complex natural products, quantification of metabolites in complex matrices (human urine and serum) using ChenomX, profiling of wine and beer spectra using MagMet etc.


Dr. David Wishart
Distinguished University Professor, Departments of Biological Sciences and Computing Science, University of Alberta Scientific Co-director, The Metabolomics Innovation Centre (TMIC) Fellow of the Royal Society of Canada (FRSC)
Dr. David Wishart (PhD Yale, 1991) is a Distinguished University Professor in the Departments of Biological Sciences and Computing Science at the University of Alberta. He also holds adjunct appointments with the Faculty of Pharmaceutical Sciences and with the Department of Pathology and Laboratory Medicine. He has been with the University of Alberta since 1995. Dr. Wishart’s research interests are very wide ranging, covering metabolomics, analytical chemistry, food chemistry, natural product chemistry, molecular biology, protein chemistry and neuroscience. He has developed a number of techniques based on NMR spectroscopy, mass spectrometry, liquid chromatography and gas chromatography to characterize the structures of both small and large molecules. As part of this effort, Dr. Wishart has led the “Human Metabolome Project” (HMP), a multi-university, multi-investigator project that is cataloging all the known chemicals in human tissues and biofluids. Using a variety of analytical chemistry techniques along with text mining and machine learning, Dr. Wishart and his colleagues have identified or found evidence for more than 250,000 metabolites in the human body. This information has been archived on a freely accessible web-resource called the Human Metabolome Database (HMDB). More recently, Dr. Wishart’s efforts have focused on using the same methods developed for the HMDB to help characterize the chemical constituents in various foods (through a database called FooDB) and food-associated biomarkers. His lab has also been using machine learning and artificial intelligence techniques to help create other useful chemistry databases and software tools to help with the characterization and identification of metabolites, drugs, pesticides and natural products. Over the course of his career Dr. Wishart has published more than 500 research papers in high profile journals on a wide variety of subject areas. These papers have been cited >100,000 times.


Dr. Rupasri Mandal
Dr. Rupasri Mandal is the TMIC (The Metabolomics Innovation Centre) node manager for the Wishart node. TMIC is Canada’s national metabolomics core facility. She obtained her Ph.D. degree in Environmental Analytical Chemistry from Carleton University, Canada. Her research experience is broad and covers the field of metabolomics, bioanalytical, biomedical, environmental/analytical and physical chemistry. Dr. Mandal has >20 years of research/work experience in these fields. Over this period of time, she has gained extensive experience in the development of novel schemes and analytical/bioanalytical techniques for targeted and untargeted metabolomics. She is quite familiar with the operation and general maintenance of all of the GC-MS, LC-MS, NMR, HPLC and sample preparation equipment in the Wishart node. She is also well acquainted with the multivariate statistical software that is used to interpret TMIC’s metabolomic results. As TMIC node manager, she is responsible for coordination and management of TMIC operations and technology developments. Dr. Mandal’s leadership and expertise are crucial for project planning and project reporting, methods and technology development and client service provision in the TMIC Wishart node. Dr. Mandal has led and participated in multiple clinical/health research projects in the Wishart node such as several maternal, prenatal, neonatal and other human disease biomarker studies. These include first trimester prediction of early-/ late-onset preeclampsia, down syndrome, fetal congenital heart defect, adult heart disease, pediatric kidney transplant and early/late-onset of osteoarthritis, lung cancer, autism, IBD and colorectal cancer.


Rafal Kantor
With over 25 years of experience as an advanced care paramedic-firefighter, Rafal Kantor is an expert in safety and emergency response. He also has excellent customer service and communication skills, as he can interact with people of all ages and backgrounds. In addition, Rafal is a seasoned marketing professional who has successfully developed and implemented marketing strategies for various projects in the residential sector. He has a deep understanding of the market trends, customer needs, and competitive landscape in the industry. He is proficient in using various marketing channels and techniques, such as online advertising, social media, email marketing, events, and referrals. He can also create compelling and persuasive sales pitches and proposals, as well as negotiate contracts and close deals. He is passionate about delivering high-quality service and value to his clients, as well as building long-term relationships with them. He is also skilled in coordinating with suppliers, vendors, customers, and other stakeholders to ensure smooth and efficient operations. He is passionate about creating innovative and effective marketing solutions that meet the needs and expectations of the target market.
Our Technology
NMR
Our affiliations with the University of Alberta and TMIC (The Metabolomics Innovation Center, ISO 17025 certified) lab give us the opportunity to access the state of the art 700 MHz NMR spectrometer (Bruker). This is equipped with a cryoprobe to further improve the signal to noise ratio thus yielding extremely high resolution and accurate quantification of the compounds present in as low as a few μM concentrations. We use Bruker’s TopSpin and Chenomx’s NMR Suite softwares to analyze, process and quantify spectra.
MS (LC and ICP)
Our lab is equipped with ScieX Triple Quad Q-Trap 5500 and Thermo Scientific TSQ Altis for the targeted LC-MS/MS analyses and Perkin Elmer NexION 350 for the ICP-MS analyses.
Automation Platforms (MagMet and LC-AutoFit)
We use a completely automated web-based platform called MagMet for fast, consistent and accurate profiling of beverage spectra (15 minutes per spectrum). We have a similar automated platform LC-AutoFit for the analyses of LC-MS data. LC-AutoFit identifies compounds through stringent matches and selection of optimal fragmentation ions from customized single ion chromatograms input by the user.
Publications
Manoj Rout, Matthias Lipfert, Brian L. Lee, Mark Berjanskii, Nazanin Assempour, Rosa Vazquez Fresno, Arnau Serra Cayuela, Ying Dong, Mathew Johnson, Honeya Shahin, Vasuk Gautam, Tanvir Sajed, Eponine Oler, Harrison Peters, Rupasri Mandal, David S. Wishart. Magn. Reson. Chem. 2023; 1–24.
Brian L. Lee, Manoj Rout, Rupasri Mandal, David S. Wishart. Magn. Reson. Chem. 2023; 1–13.
Magmet Wine (manuscript in preparation)
Contact Us
Got a technical issue? Need details about our analytical services? Let us know!
info @ amtianalytics.ca